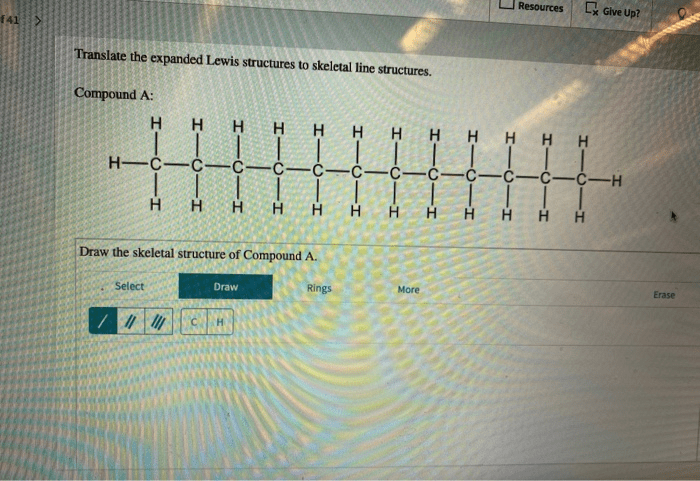
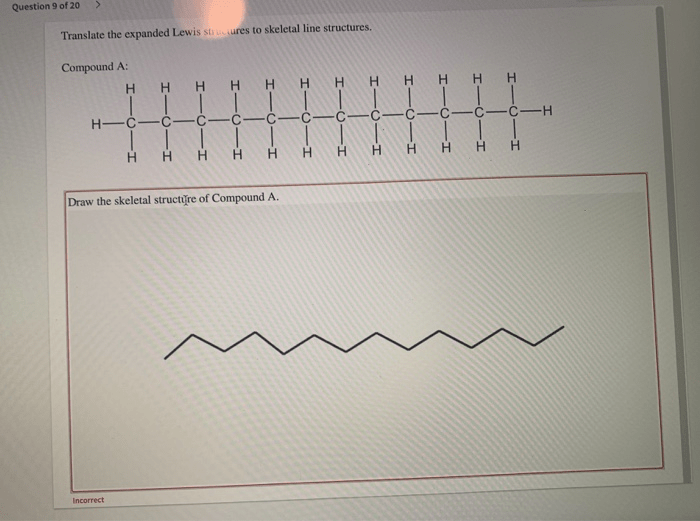
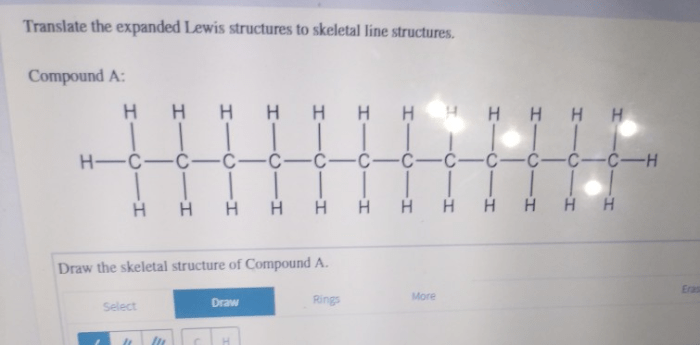
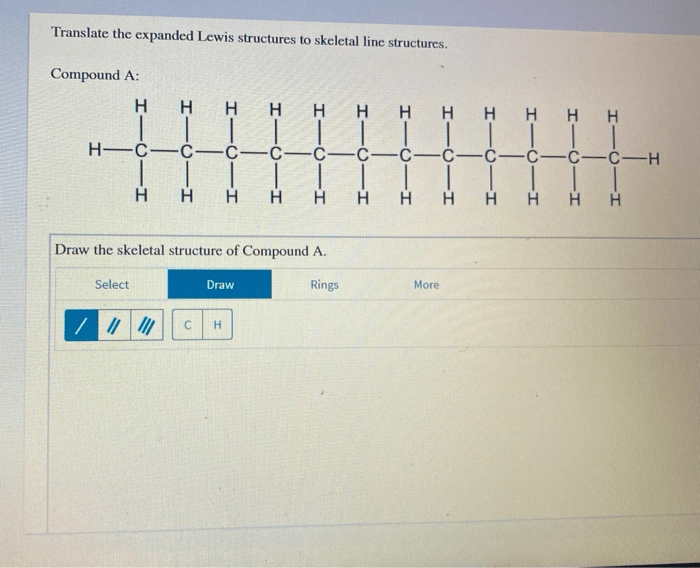
Translate the expanded lewis structures to skeletal line structures. – Translate expanded Lewis structures to skeletal line structures is a fundamental skill in chemistry that allows us to represent molecular structures in a simplified and concise manner. This process involves converting the detailed representation of a molecule’s bonding and electron distribution, known as an expanded Lewis structure, into a skeletal line structure that captures the essential connectivity and bonding information.
Understanding the translation between these two representations is crucial for visualizing molecular structures, predicting their properties, and comprehending chemical reactions. In this comprehensive guide, we will delve into the concept of expanded Lewis structures, explore the nature of skeletal line structures, and provide a step-by-step process for translating between the two.
Understanding Expanded Lewis Structures
Expanded Lewis structures represent molecules by showing all the valence electrons as lone pairs or bonds. They provide a detailed representation of the electron distribution within a molecule, including non-bonding electrons.
Expanded Lewis structures are useful for understanding the electronic structure of molecules, predicting molecular shapes, and determining the reactivity of molecules.
Skeletal Line Structures
Skeletal line structures are simplified representations of molecules that use lines to represent bonds and vertices to represent atoms. They are commonly used to represent organic molecules, which are molecules that contain carbon.
In skeletal line structures, carbon atoms are not explicitly shown, but they are assumed to be at the vertices of all lines. Hydrogen atoms are also not explicitly shown, but they are assumed to be bonded to the carbon atoms.
Translating Expanded Lewis Structures to Skeletal Line Structures, Translate the expanded lewis structures to skeletal line structures.
To translate an expanded Lewis structure to a skeletal line structure, follow these steps:
- Identify the carbon atoms in the expanded Lewis structure.
- Draw a line for each bond between the carbon atoms.
- Add a hydrogen atom to each carbon atom that has less than four bonds.
For example, the expanded Lewis structure of ethane (C 2H 6) is:
The skeletal line structure of ethane is:
Questions and Answers: Translate The Expanded Lewis Structures To Skeletal Line Structures.
What is the purpose of translating expanded Lewis structures to skeletal line structures?
Translating expanded Lewis structures to skeletal line structures simplifies complex molecular representations, making them easier to visualize and analyze. Skeletal line structures focus on the connectivity and bonding of atoms, providing a concise overview of the molecular structure.
How do I translate an expanded Lewis structure to a skeletal line structure?
To translate an expanded Lewis structure to a skeletal line structure, follow these steps: 1) Remove all lone pairs of electrons. 2) Replace each pair of bonding electrons with a single line. 3) Remove all hydrogen atoms and their associated lines.
4) For carbon atoms, add enough lines to satisfy the octet rule.